Feature your business, services, products, events & news. Submit Website.
Breaking Top Featured Content:
Novavax Launches ‘Phase 3’ COVID-19 Vaccine Trials As UK, France Post Record Numbers: Live Updates
Tyler Durden
Thu, 09/24/2020 – 16:26
Summary:
- Novavax becomes 5th US project to launch Phase 3 vaccine trials
- California cases top 3k again
- Ireland tightens social distancing rules for 2nd time in a week
- France reports record 16k+ new cases
- UK breaks record for most new daily cases
- Spain reports 3,471 new cases
- New York sees hospitalizations north of 500
- Trump vetos vaccine restrictions
- China says mass vaccinations to take up to 2 years
- France introduces more curbs
- Israel lockdown intensifies
- FDA delivers emergency authorization
- Moscow reports 1,050 new cases
- Indonesia suffers 2nd straight record
* * *
Update (1615ET): Novavax has just become the latest company to announce the start of ‘Phase 3’ trials for its COVID-19 vaccine candidate. It’s officially the 5th US company to announce the start of Phase 3 trials, just days after Johnson & Johnson.
Here’s the press release:
Novavax, Inc. (Nasdaq: NVAX), a late stage biotechnology company developing next-generation vaccines for serious infectious diseases, today announced that it has initiated its first Phase 3 study to evaluate the efficacy, safety and immunogenicity of NVX-CoV2373, Novavax’ COVID-19 vaccine candidate. The trial is being conducted in the United Kingdom (UK), in partnership with the UK Government’s Vaccines Taskforce, and is expected to enroll and immunize up to 10,000 individuals between 18-84 (inclusive) years of age, with and without relevant comorbidities, over the next four to six weeks.
“With a high level of SARS-CoV-2 transmission observed and expected to continue in the UK, we are optimistic that this pivotal Phase 3 clinical trial will enroll quickly and provide a near-term view of NVX-CoV2373’s efficacy,” said Gregory M. Glenn, M.D., President, Research and Development at Novavax. “The data from this trial is expected to support regulatory submissions for licensure in the UK, EU and other countries. We are grateful for the support of the UK Government, including from its Department of Health and Social Care and National Institute for Health Research, to advance this important research.”
NVX-CoV2373 is a stable, prefusion protein made using Novavax’ recombinant protein nanoparticle technology that includes Novavax’ proprietary MatrixM™ adjuvant. The vaccine has a favorable product profile that will allow handling in an unfrozen, liquid formulation that can be stored at 2°C to 8°C, allowing for distribution using standard vaccine channels.
Novavax has continued to scale-up its manufacturing capacity, currently at up to 2 billion annualized doses, once all capacity has been brought online by mid-2021.
About the Phase 3 Study
Consistent with its long-standing commitment to transparency and in order to enhance information-sharing during the worldwide pandemic, Novavax will be publishing its UK study protocol in the coming days.
The UK Phase 3 clinical trial is a randomized, placebo-controlled, observer-blinded study to evaluate the efficacy, safety and immunogenicity of NVX-CoV2373 with Matrix-M in up to 10,000 subjects aged 18 to 84 years. Half the participants will receive two intramuscular injections of vaccine comprising 5 µg of protein antigen with 50 µg Matrix‑M adjuvant, administered 21 days apart, while half of the trial participants will receive placebo.
The trial is designed to enroll at least 25 percent of participants over the age of 65 as well as to prioritize groups that are most affected by COVID-19, including racial and ethnic minorities. Additionally, up to 400 participants will also receive a licensed seasonal influenza vaccine as part of a co-administration sub-study.
The trial has two primary endpoints. The first primary endpoint is first occurrence of PCR-confirmed symptomatic COVID-19 with onset at least 7 days after the second study vaccination in volunteers who have not been previously infected with SARS-CoV-2. The second primary endpoint is first occurrence of PCR-confirmed symptomatic moderate or severe COVID-19 with onset at least 7 days after the second study vaccination in volunteers who have not been previously infected with SARS-CoV-2. The primary efficacy analysis will be an event-driven analysis based on the number of participants with symptomatic or moderate/severe COVID-19 disease. An interim analysis will be performed when 67% of the desired number of these cases has been reached.
Source: Novavax
* * *
Update (1530ET): California reported more than 3,000 new coronavirus cases for the seventh time in eight days on Thursday, as well as reporting more than 100 deaths back-to-back for the first time in a week.
Another 3,170 people tested positive for the disease over the past 24 hours, up from 3,146 from the prior day, and compared with 3,238 a week ago.
With the exception of 2,630 new infections reported on Wednesday, California health authorities have reported more than 3,000 daily infections for seven of the past eight days.
The death toll rose by 110, down from Wednesday’s one-week high of 133 and compared with 106 last Thursday. This comes days after the state passed 15,000 deaths.
Ireland, meanwhile, has tightened coronavirus restrictions for the second time in less than one week, imposing tough new measures on people and businesses in county Donegal as it struggles to contain rising infection rates.
* * *
Update (1425ET): France just followed up the UK by reporting a record jump in new COVID-19 infections as new social-distancing measures are just taking effect.
French health officials reported 16,096 new cases on Thursday, bringing the total to 497,237, the French Public Health Agency said. In a sign that increased testing isn’t entirely to blame, the countrywide positivity rate for the day climbed to 6.2%.
Meanwhile, the number of COVID-19 deaths increased by 52 to a total of 31,511.
* * *
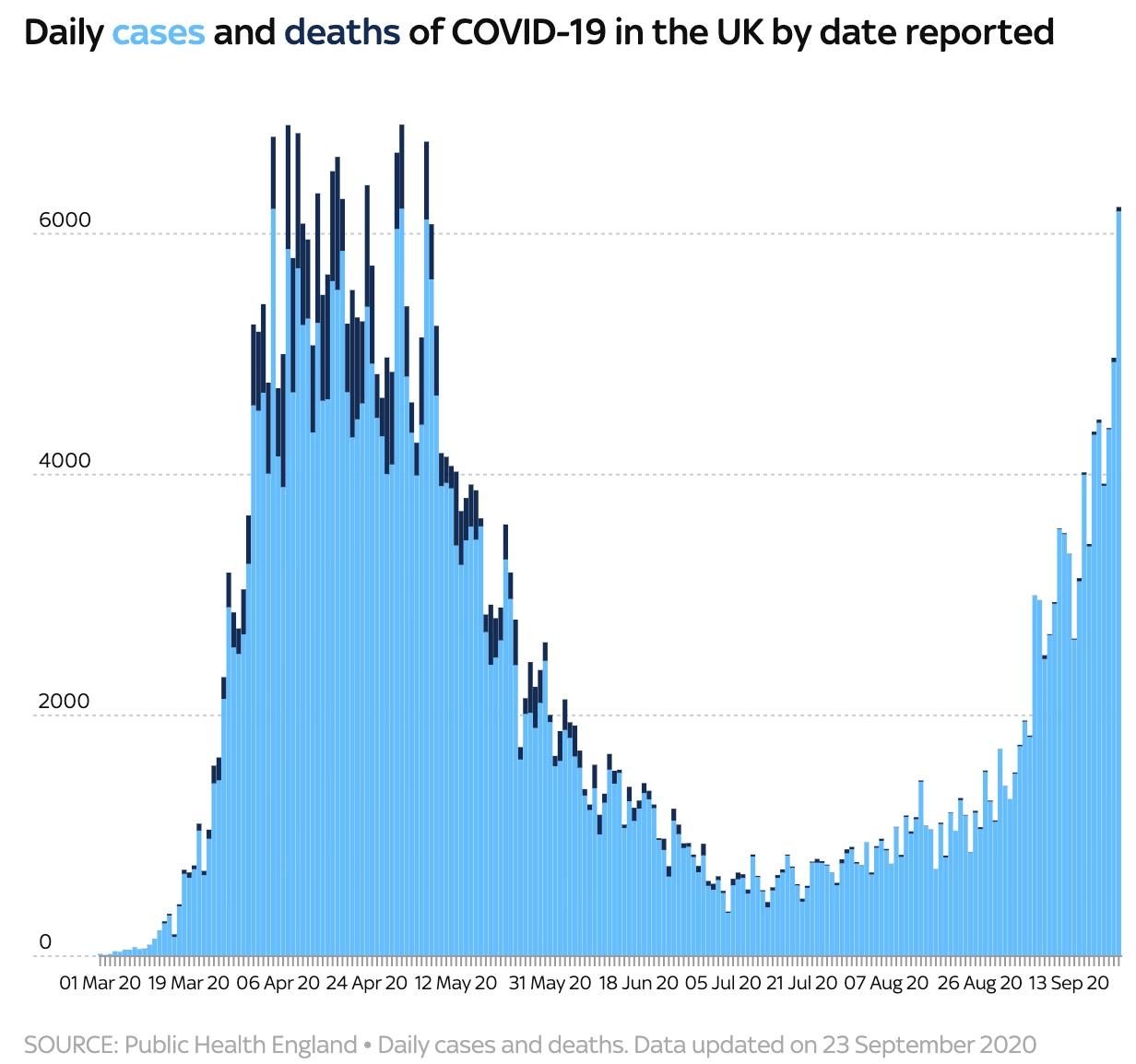
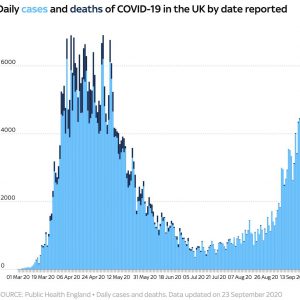
Update (1215ET): With the UK government ramping up testing in keeping with BoJo’s promise to take more steps to combat a turnaround in infection rates, the UK has just reported its largest-ever daily tally.
6,634 new cases were reported, the most in a single day since the pandemic began. 40 new deaths were also reported.
Despite this new peak, epidemiologists told Sky News that realistically, these numbers are nowhere near ‘the peak’ reached in the spring, when as many as 100k people may have been infected per day.
Since the end of August, however, daily new cases have climbed 180%.
Elsewhere, COVID-19-linked hospitalizations in New York state have broken above 500, the highest tally since Aug. 19. All of this comes as cases in the US continue a post-LDW spike, while daily deaths also increase.
Here’s an update on where things stand in the US.
In other European news, Spain just reported 3,471 new cases of COVID-19, compared with 4,143.
* * *
While the US marches toward the 7 million mark, France announced new restrictions on Wednesday calling for bars and restaurants to be shuttered in the ‘hot spot’ of Marseille, and Germany added 11 regions to its list of COVID hotspots, as the second wave of COVID-19 infection spreads across Europe.
China has been pressing ahead with its vaccination projects, with multiple efforts already well into ‘Phase 3’ testing. But if China’s COVID-19 infection rates are really so low as to be virtuallly nonexistent, as Chinese official data, and state-controlled media reports, have suggested, then why is the Communist Party of China attaching such a high priority to the country’s domestic mass-vaccination efforts?
A top government scientist told the local press that it’ll take China up to two years to finish vaccinations on a mass scale, said infectious disease expert Zhong Nanshan, who was speaking at an “industry event”, according to China’s Changjiang Daily.
One could argue that another outbreak is just around the corner, but after months of Beijing’s heavy handed “wartime footing” approach, people in Wuhan are partying like its 2019, and even a handful of domestic cases can trigger ‘localized’ lockdowns that can seal off cities from their surrounding province.
Circling back to the US, President Trump threatened to veto any attempt to tighten rules related to emergency clearance of a vaccine, a statement that will inevitably trigger another round of accusations about Trump meddling with government scientists and applying undue political pressure that could compromise the safety, and credibility, of the eventually-approved vaccine.
Still, Dr. Fauci and Dr. Redfield told Congress during yesterday’s hearing that they “wouldn’t hesitate” to get a vaccine if one was offered.
One day after JNJ became the fourth US vaccine project to enter ‘Phase 3’ testing, AstraZeneca said it is still waiting for a decision from the FDA on whether it can resume tests in the country after halting global trials due to concerns about a participant who became ill in the UK.
Though case numbers across Sweden remain well below their springtime peak, a recent surge in cases in and around Stockholm has prompted the country’s top health officials to consider imposing new localized restrictions to prevent a broader resurgence. Swedish PM Lofven said the country “would not hesitate” to take further action to limit the spread.
After separately announcing new measures earlier in the week, Spain, France, the UK and Germany are leading European nations in combating a second wave that continues to rise. With Europeans opposed to a lockdown return, all of these countries are relying on ‘localized’ restrictions – particularly on bars and restaurants, and large gatherings and weddings – as their front-line of defense, with leaders (notably Johnson) warning that the restrictions could remain in place for up to six months.
The biggest numbers out of the US yesterday came from Texas, which followed California to become the 4th state to see its death toll top 15,000.
Here’s a rundown of important numbers from yesterday, accurate as of 0630ET:
31,914,770: confirmed cases worldwide
977,109: confirmed deaths worldwide
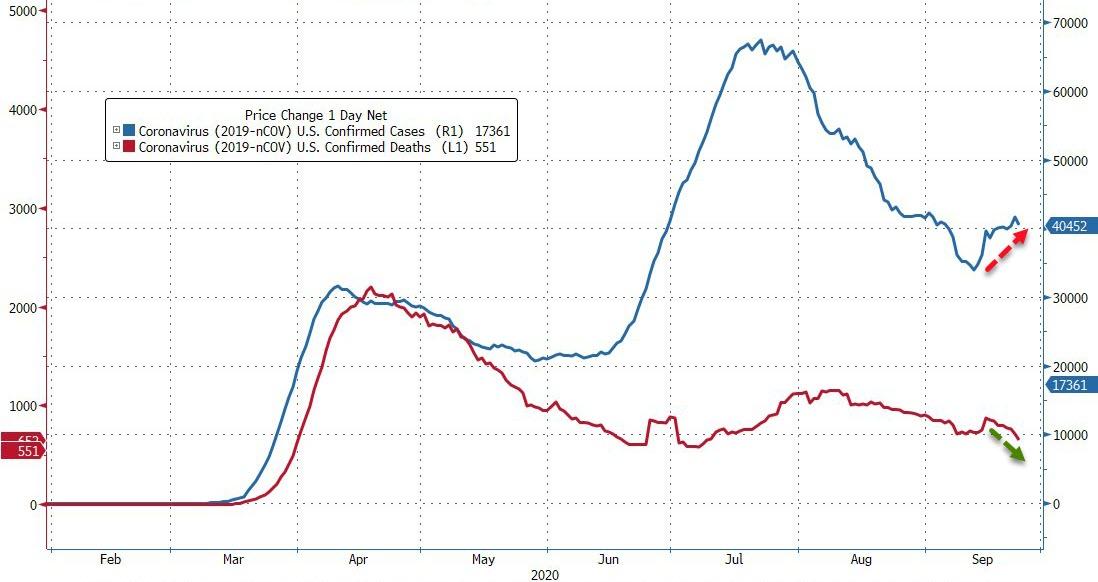
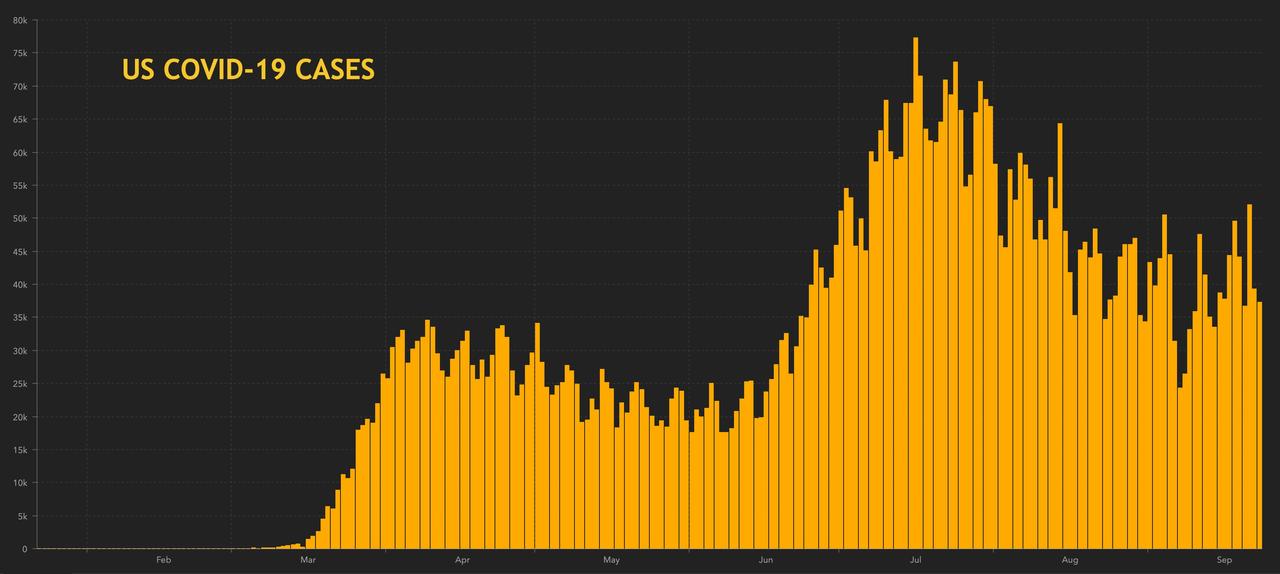
37,330: New US cases recorded yesterday
6,935,415: Total cases confirmed in the US
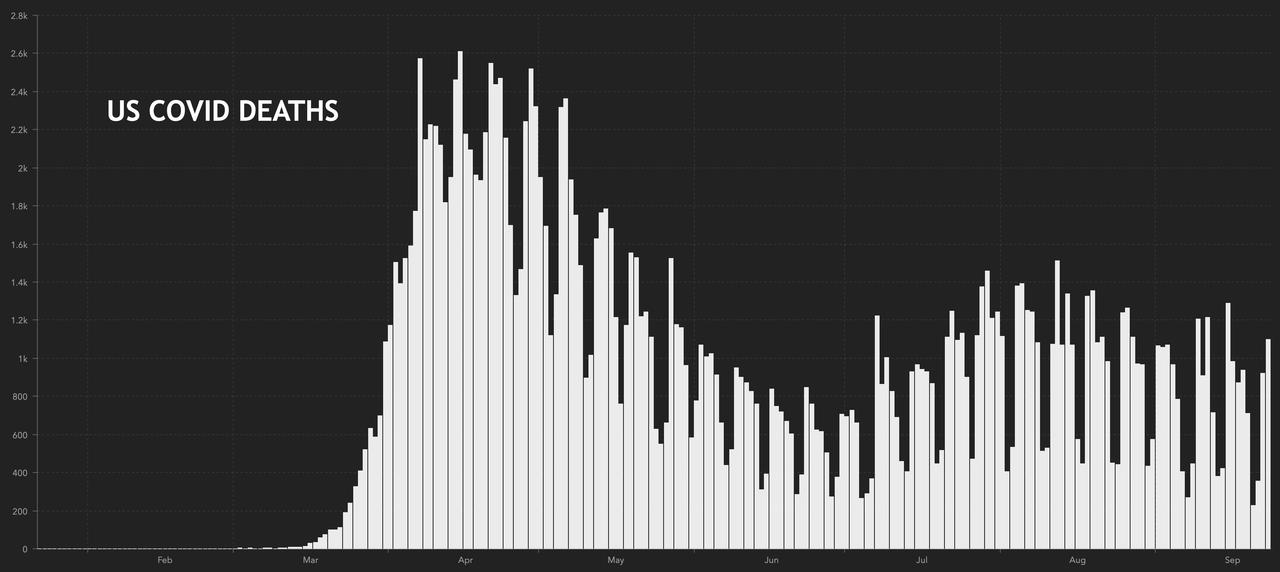
1,098: deaths in the US recorded yesterday
201,920: total U.S. deaths
97,459,742: tests conducted in the U.S.
Cases have fallen from a five-week high reached earlier in the week…
…while the US saw the highest number of deaths in a week yesterday.
Here’s other important news from overnight:
US FDA delivered emergency authorization for the first serology/antibody point-of-care COVID test (Newswires).
China’s Sinovac Biotech hopes to supply its experimental vaccine across Latin America as quickly as possible by outsourcing some manufacturing procedures to a partner in Brazil. Sinovac plans to provide semi-finished products to its partner Instituto Butantan, which will complete the rest of the process and supply finished items to other South American countries, Chairman Yin Weidong said at a news conference, part of China’s effort to ‘atone’ for unleashing SARS-CoV-2 on the world (Source: Nikkei).
Indonesia reports a daily record high for the second consecutive day with 4,634 new infections, and 128 deaths. The country has now a total of 262,022 coronavirus cases, with the death toll crossing the 10,000 mark for the first time to 10,105 (Source: Nikkei).
The Philippines reports 2,180 new infections and 36 additional deaths. Total confirmed cases rose to 296,755, still the highest in Southeast Asia, while deaths reached 5,127, nearly half of which were recorded in the past 30 days (Source: Nikkei).
SoftBank Group starts offering PCR coronavirus testing with saliva that will cost 2,000 yen ($19) per person, excluding delivery fees, for corporate customers. In Japan, PCR testing is typically priced from 20,000 to 40,000 yen. The Japanese tech investor aims to expand the testing market by making tests available to people without symptoms at a reasonable price through its unit. It also plans to offer the service to individuals this winter (Source: Nikkei).
China reports seven new coronavirus cases for Thursday, down from 10 reported a day earlier. All new cases were imported infections involving travelers from overseas. The number of new asymptomatic cases rose to 20 from 18 a day earlier (Source: Nikkei).
Moscow registered 1,050 new cases in the last day, the first time that the Russian capital diagnosed over a thousand infections since June. New daily cases in Moscow have grown by two-thirds since Sept. 1, when schools opened nationwide. The number of infections is rising throughout Russia, with 6,595 new cases in the last day. There have been 1,128,836 reported infections, the fourth highest in the world, after the U.S., India and Brazil (Source: Bloomberg).
The Israeli government tightened lockdown restrictions for the next two weeks to try and fight a coronavirus outbreak that’s spun out of control. Just last week, the government imposed its second lockdown since the pandemic began. With daily new infections surging dramatically, the government voted early Thursday to clamp down further during a season of major Jewish holidays by almost totally idling the private sector, allowing only essential employees to work (Source: Bloomberg).
Russia is preparing to supply 17 more countries with its Avifavir COVID-19 treatment (Newswires).
Continue reading at ZeroHedge.com, Click Here.