Feature your business, services, products, events & news. Submit Website.
Breaking Top Featured Content:
The Race To Save Lives: Comparing Vaccine Development Timelines
Major advancements in medicine have led to a significant increase in average life expectancy, with vaccines being hailed as one of the most successful interventions to date.
In fact, the World Health Organization estimates that vaccines have prevented 10 million deaths between 2010 and 2015 alone. But while some were created and distributed in just over four months, others have taken over 40 years to develop. Then again, previous pandemics have petered out without any vaccine at all.
With approved COVID-19 vaccines soon to be distributed across the globe, the vaccine development process is being scrutinized by experts (and non-experts) the world over.
In the graphic below, Visual Capitalist’s Katie Jones explores how long it has historically taken to bring a vaccine to market during pandemics dating back to the 1900s, and what the process entails.
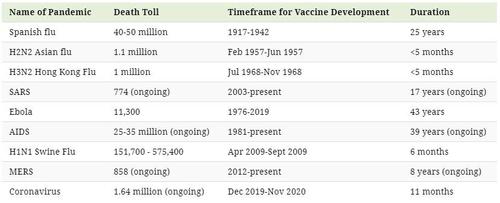
Pandemic Vaccines of the Past
Although the assumption can be made that developing a vaccine for infectious diseases has become more efficient since the 1900s, that statement is not entirely correct.
It took approximately 25 years to develop a vaccine for the Spanish Flu which killed between 40-50 million people. Similarly, it was only last year that the FDA approved the first Ebola vaccine—an effort that took 43 years since the discovery of the virus.
But while scientists and medical experts have made headway in stopping major pandemics in their tracks, some of the worst outbreaks in history have yet to be cured.
Here is a closer look at the timeframes for vaccine development for every pandemic since the turn of the 20th century:
When it comes to the speedy development of a COVID-19 vaccine, funding has played a vital role. With case numbers growing at an alarming rate, demand and urgency for a vaccine are high. In the U.S., the government paid Pfizer and BioNTech almost $2 billion for 100 million doses of a safe vaccine for COVID-19. This level of support from governments the world over means that pharmaceutical giants have less financial uncertainties to deal with compared to other vaccines.
Even though the global endeavor to distribute COVID-19 vaccines is now underway, many experts are concerned that the pace of approval could compromise long-term safety—but there are rigorous steps a vaccine must first go through before it is approved.
The Journey of a Vaccine Candidate
On average, it takes 10 years to develop a vaccine. According to the CDC, there are six stages involved in the process from start to finish:
-
Exploratory stage: This stage typically consists of basic lab research that can last anywhere from 2 to 4 years.
-
Pre-clinical stage: This stage uses tissue-culture or cell-culture systems and animal testing to give researchers an idea of how humans might respond to a candidate vaccine.
-
Clinical development: Within the clinical development stage, there are three phases. Phase 1 examines the response of a small group of people to a candidate vaccine. Phase 2 involves giving the candidate vaccine to a larger group of people to study its safety, immunogenicity, proposed doses, schedule of immunizations, and method of delivery. In Phase 3, the vaccine is given to thousands of people to further test for efficacy and safety.
-
Regulatory review and approval: National Regulatory Authorities are responsible for the approval of vaccines in different countries. For example, the U.S. Food and Drug Administration’s Center for Biologics Evaluation and Research (CBER) regulates all U.S. vaccines.
-
Manufacturing: Typically, it can take anywhere from 6 to 36 months to produce, package, and deliver a high quality vaccine.
-
Quality control: Different batches of a vaccine are continuously tested by different authorities around the world to ensure its ongoing safety.
Despite these lengthy timeframes, the COVID-19 vaccines and subsequent candidates have overturned the conventional process due to their unconventional technology.
Innovative Technologies Driving COVID’s Cure
Even though there are no approved vaccines for other coronaviruses such as MERS and SARS, previous research into these diseases has helped identify potential solutions for COVID-19 using messenger RNA (mRNA) technology.
“The mRNA vaccine platform technology [which the Pfizer/BioNTech vaccine uses] has been in development for over two decades.”
– Dr Zoltán Kis, Imperial College London.
The technology instructs our bodies to produce a small part of the COVID-19 virus called a spike protein. This triggers the immune system to make antibodies to fight against it and prepares the body for an actual COVID-19 infection.
Containing COVID-19 Batch-by-Batch
Deployment of a safe and effective vaccine could have the potential to save millions of lives and prevent infection for many more.
Although some experts have criticized the speed of vaccine candidate approvals, the quality will be closely monitored on a batch-by-batch basis.
With the COVID-19 crisis showing no signs of slowing down, most of us continue to live in hope that the light is at the end of the tunnel.
Tyler Durden
Thu, 12/24/2020 – 04:15
Continue reading at ZeroHedge.com, Click Here.